Abstract
Introduction
The optimal induction and conditioning regimen for patients with transplant eligible mantle cell lymphoma (MCL) remains unknown. Common approaches include the Nordic MCL2 regimen (rituximab (R), cyclophosphamide, vincristine, doxorubicin, prednisone (R-maxi-CHOP)) alternating with high-dose cytarabine (Geisler, Blood 2006) and R-Hyper CVAD/R-MA (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with high dose methotrexate/cytarabine) (Romaguera JCO 2005). The aim of this study was to compare the efficacy of different induction and conditioning regimens in transplant eligible MCL patients.
Methods
We performed a multi-center retrospective analysis of MCL patients treated with induction chemo-immunotherapy and deemed fit enough for autologous stem cell transplant (ASCT) between December 2001 and December 2015. Patients were excluded if they did not receive rituximab with induction chemotherapy. Transplant eligibility was determined by the treating physician and patients were included if fit enough to undergo ASCT regardless of whether it was subsequently performed. Patient characteristics, prognostic factors and outcomes following treatment were collected. We used appropriate descriptive statistics to compare baseline characteristics between treatment groups, and calculated progression free (PFS), overall survival (OS) and follow up from date of diagnosis to date of disease progression or death from any cause, or death from any cause, respectively. Patients alive at last observation were censored. We used univariate Cox regression to identify associations between prognostic factors and outcomes, and covariates with P-values <0.1 were included in a Cox multivariate regression.
Results
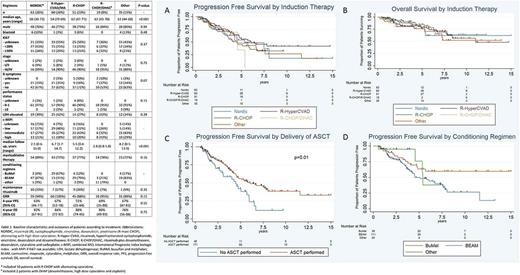
We identified 228 patients and grouped them according to induction regimen received. Their baseline characteristics, treatment and outcomes are summarized in Table 1. Overall, the 5 groups were mostly balanced in terms of baseline characteristics. Patients who underwent Nordic-like and Hyper-CVAD regimens were younger than the other groups. After a median follow up of 4.2 years (range 0.4-15), among the entire cohort the 4-year PFS was 62% (95%CI 54-69%) and OS was 84% (95%CI 78-88%). By univariate analysis, c-MIPI (combined MCL International Prognostic Index biologic index, with MIPI if Ki67 was not available), B symptoms, stage and bone marrow involvement at diagnosis were significantly associated with PFS; c-MIPI, blastoid histology, and B symptoms for OS.
Induction chemo-immunotherapy regimen was not associated with PFS (Fig A) or OS (Fig B) by multivariate analysis and only c-MIPI high-risk group (HR=1.8 [95%CI 0.9-3.7], P=0.09) showed an adverse trend for PFS and similarly for OS (HR=2.7 [95%CI 1.1-6.4], P=0.02). Cytarabine-containing induction regimens were not significantly associated with PFS (HR=0.9 [95%CI 0.5-1.6], P=0.72) or OS (HR=1.5 [95%CI 0.7-3.0], P=0.27) by multivariate analysis. Considering only patients who received an ASCT, induction therapy was still not associated with PFS (P=0.81) or OS (P=0.51).
Among patients who responded to induction and did not experience disease progression within 6 months of diagnosis, delivery of ASCT was associated with favorable PFS (P=0.01) (Fig C) and OS (P=0.07) by univariate analysis. PFS (HR=1.3 [95%CI 0.8-2.0], P=0.19) (Fig D) or OS (HR=0.9 [95%CI 0.5-1.6], P=0.75) were not affected by conditioning regimen among the same cohort. Use of the BEAM (carmustine, etoposide, cytarabine, melphalan) conditioning regimen was associated with inferior PFS relative to BuMel (busulfan and melphalan) (HR=2.0 [95%CI 1.1-3.6], P=0.02) but not OS (HR=1.1 [95%CI 0.5-2.3] P=0.77). Maintenance rituximab among transplanted patients was associated with neither PFS (P=0.94) nor OS (P=0.46) but numbers treated were low.
Conclusion
Within the limits of a retrospective study and modest power for some comparisons, induction therapy did not influence ORR, PFS or OS. BuMel conditioning may result in superior disease control. Large prospective studies are required to define the optimal chemo-immunotherapy induction and conditioning regimen for MCL patients.
Bishton: Roche: Other: Travel Sponsorship. Ritchie: Amgen Inc.: Honoraria. Schwarer: Novartis: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Roche: Honoraria; Amgen: Honoraria; Specialised Therapeutics: Honoraria. Tam: Janssen Cilag: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Roche: Honoraria, Research Funding. Opat: Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Beigene: Research Funding; Mundipharma: Consultancy; Amgen: Research Funding. Hawkes: Roche: Other: Travel expenses; Bristol-Myers Squibb: Research Funding; Roche: Other: Travel expenses, Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Other: Travel expenses; Merck Serono: Research Funding; Merck Sharpe Dohme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal